Common Objectives: Josh Scott MFA Thesis Show at Utah State University (2021)
Earlier this year, grad students Josh Scott, Ayla Murray and Matt Sloan completed their MFAs here at Utah State University. They all put on fabulous shows and I felt compelled to document them. Over the next few days, I will be posting about each one.
First up is Josh Scott and his show, “Common Objective.” Josh Scott’s pottery is some fine stuff. Hope you enjoy the pictures, but before we get to that, here’s the context for the exhibition…
Hailing from Florida, and back living there now, Josh Scott makes fabulous wood-fired pots. He has a lovely touch with clay, leaving just enough marks from the making process to keep the pots feeling fresh.
I’ve had the pleasure of helping Josh fire over the past year and saw most of these pots come out of the kiln. There is a big difference in seeing the pots fresh from the kiln and then all set up in the gallery. A lot of sanding and cleaning goes on before they make it to the pedestal.
Josh is a meticulous maker as well as cleaner of pots. It was wonderful to walk through the gallery see them all arranged. I’m happy to say that I snagged a couple for my own collection.
Here’s how the gallery looked from each end…
Now let’s get in to the pots. First up I want to show some of Josh’s teapots…




Here are a nice collection of wall-hung dishes…







Here is a larger pot that was fired right in the front of “Bob,” one of the train kilns here at USU. It has quite a spectacular surface…






Here’s close up pictures of some of the other pots in the show…
I saved some ewers for the end. These are some of Josh’s favorite pots to make and it shows! I have one in my kitchen full of soy sauce right now…
Check out his website here: https://www.joshscottpottery.com/
Test Soda Firing #3 (10/12/20)
For my third soda firing, I wanted to re-test all the clay bodies I had made for my second firing in a different atmosphere. I had seen what they looked like in a lightly reduced atmosphere, but was curious what would happen if I again ignored body reduction but reduced the kiln hard during the soda introduction.
I also took the opportunity to try and hone in on some of the successful clay tests by making some more. I mixed up 7 more stoneware clays (50lbs each) and 5 more porcelain test bodies (10lbs each). I threw these into the same shapes as my original tests: trimmed bowls and cups. I had a few orders to fill so I hoped for the best and made them out of these untested bodies too. I also threw a few flower pots to help fill up the kiln.
For this firing I pre-mixed my soda using Gail Nichols’ recipe. 50% whiting, 25% light soda ash and 25% baking soda (15lbs soda : 15lbs whiting). Supposedly the whiting helps separate the soda particles so they can vaporise more effectively. I had to take dense soda ash and heat it to 600°F in an electric kiln to convert it to light soda ash. I mixed the dry ingredients first and then added water. As I mixed the solution with an electric drill it started to turn slushy and then solid at a certain point. It was very much like making plaster. I spooned this mixture out into egg cartons as you can see below. Mixing the soda this way was super easy. It took much less time to introduce than spraying.
I would chuck in half an egg box in each side along with several pieces of wood, and then pushed the damper in to 3". I also turned the air and gas down a little during this soda period. I took my time with the soda introduction, starting at cone 7 and continuing up to cone 10/11. After introducing the soda and wood, I kept the kiln in pretty heavy reduction for half an hour (during which time the temperature crashed about 150 degrees) and then I opened the damper back up to 6” to regain the temp for half an hour. So these cycles were an hour long (5/6 hours of soda all together). I think I could maybe cut this down but it made it easy to manage, coming out every half hour to adjust the kiln.
Here are some pictures from the unload…
RESULTS
I sort of hoped that with this firing, I would get all the answers. That was not the case! I came away with more questions than answers… again. But I guess that’s why it’s so fascinating.
I packed the kiln tighter than my second firing (old habits die hard) and this made for some dry areas.
The top and bottom of the kiln got lots of soda, but the middle was pretty dry. To remedy this next time, I plan to pack the top and bottom tighter and leave the middle more open. Also just cramming less pots in.
The heavy reduction during the soda introduction made for quite a bit of carbon trapping… mostly up in the top of the kiln. There were lots of darker greys up there whereas down low, it was a lighter soda build up. Down low, I got more grey/black specks on a white surface rather than up high, where it was lighter grey spots on a darker grey surface.
I found the darker heavily carbon-trapped surfaces from the top of the kiln to be a bit too contrasty with the orange flashing. I don’t think I want to reduce this heavy during soda introduction going forward.
Here are some of the pots I found most interesting from my first couple of firings…
I organised the tests and put them to bed until I get back from the holiday break. Excited to get stuck in to my fourth soda firing in January of February. But for now, it’s time to read some books and relax!
Hope you all have a lovely holiday and here’s to a better 2021!
Test soda firing #2 (27/11/20)
In my first soda firing, I learnt a lot about the kaolins and other clays in the magic cave of materials at USU. On the basis of my test tiles, which you an see in my last blog post, I was able to make some educated guesses about which materials I wanted to include in test clay bodies.
I set about making 50lb batches of 8 white (or at least pale) stoneware blends, and 10lb batches of 11 different porcelain blends. Making the stoneware bodies was easy in the little bluebird mixer at USU; I was able to make all 8 of the stoneware clays in an afternoon and bag them up to sit a week or so before I threw them.
The porcelains were mixed dry in separate 5 gallon buckets. I added water and let them sit to absorb without mixing overnight. I added water until they were the consistency of thick cream, throughly blunged them and set them out to dry in plaster beds lined with clean sheets. They took maybe 3/4 days to dry to the point that I could take them out and make them into coils to finish drying up. This process was fairly time-consuming and I had to be really on top of how the porcelains were drying. Keeping track of which blend was which was also a challenge. I marked the plaster beds and sheets but there was some head scratching nonetheless.
Next I decided to make several yunomi (cups) with each clay and a couple of wide open bowls too. The idea was to make forms with trimmed feet in order to give me more information about how the clays operated and looked. I also decided to set the bowls on their sides in the kiln to encourage interesting soda flashing, and more importantly, to see if the clays would warp. Again, keeping track of the 19 different clays as I was making was a challenge; I had a very technical system of torn slips of paper reading “14” or “3” that sat on my ware boards next to the thrown forms.
The system worked, but was not foolproof. Unfortunately, I am a fool. Once or twice, the little slip of paper got shisked off my board of pots by a gust of wind or a stray corner of a sheet of plastic. At these moments, I had to use my limited powers of logic to figure out what the clays were and re-label them. Shockingly I managed to get all the pots trimmed and labelled without major confusion or tears.
I had more of each of the stoneware clays so threw some extra pots with these, and experimented with a few slips on them too. This helped fill the kiln. I packed the kiln looser this time, trying to put larger pots in the center of shelves with smaller pots towards the edges.
From my first firing, I did not know how much of an impact the reduction cooling had, so this time I aimed at an oxidised firing. I wanted to see if my slips/new clays would be totally bland without any reduction. It felt kind of wrong to be firing in this way, just ignoring body and glaze reduction, and cooling the kiln naturally in oxidation. But it was an experiment.
Alright, here’s the results… (I will discuss the results below the pictures).
RESULTS
First thing of note was that the pots were not oxidised. This is what I had been aiming at, so it was a little confounding at first. In retrospect I didn’t pay enough attention to the level of gas entering the kiln. I kind of thought that if I did not intentionally reduce the atmosphere then it would be oxidised or neutral. This was clearly not true. I think to truly oxidise the kiln I would have to fire over a longer period with less gas and more air throughout.
However… the results were pretty nice, even though it did not go as planned. What did I learn? It is way easier to reduce pots in this kiln than expected. I think body reduction is unecessary, but will further investigate this to make sure.
In general the colors were brighter than in my first test firing but there was less drama on the surface of the pots. There were less deep red tones, but also perhaps less distinct flashing lines. Somewhat softer flashing and softer surfaces in general.
I used 15lbs of soda. The first 5 went in as spray, but I got fed up with how long it was taking and my nozzle was acting up (I partially melted it haha), so decided to make some burritos and shove them in. I mixed damp soda, baking soda and rice hulls and wrapped them in newspaper to introduce them.
The soda went in over 3/4 hours this time instead of 6 hours in the first firing. Not a much different sodarisation level on the pots… I think because the gas and air pressure was pretty high and I didn’t crank the damper in as much. Also less carbon trapping. And less blue glassy drips off the shelves onto pots. Had a lot of that in my first firing. Could have been partly due to the shelves not having been cleaned as thoroughly before I fired that first time.
The clay tests proved very interesting. There were significant differences in how they flashed, how glassy and vitrified they were, how much they shrank. Some excellent information. Some of the test bodies clearly were not going to work; a couple of them warped really badly or cracked.
It was clear from having at least a cup and bowl of each clay that the placement in the kiln was crucial to the resulting surface of the pot. This leads me to realise I need to put these same test bodies in several more firings to see how that affects the results.
Below are a few examples of a few of my clay body tests. The first had some strange cracking on the bottom. I think it was from not having wedged the clay thoroughly enough. You can kind of see it on the base here. I was perhaps a little fast and loose when prepping and making this particular bowl.
Here’s a pitcher that came out well, but unfortunately this particular clay body cracked quite a bit where thin.
This final one is a plate that came out well I thought; nice surface variation and mark around the wadding.
FIRING SCHEDULE…
That’s all for now. Stay tuned though, I will be posting reults from my #3 soda firing soon. Thanks for reading!
Test Soda Firing #1 (8/11/20)
Lauren and I moved to Logan, Utah in July for me to start an MFA in Ceramics at Utah State University. My main goal here is to experiment with local materials; especially using local rocks to make glazes. I have been out to collect some samples, but before I dive into that research, I wanted to test the myriad of purchasable clays that the studio has for our use.
In pottery, clay is number 1. It is my main ingredient. The tomatoes in the tomato sauce. In North Carolina, I was making my own clay, a blend of four or five different local clays. But there isn’t a ton of local sources of clay in Utah. Plenty of rocks, but not much clay, really. Whilst I am here, I feel okay trying out these commercial clays and not going mad trying to harvest and process local clay, too. There are several rooms full of different kaolins and all sorts that I have access to. It’s a potters playground!
The system is very well set up at USU. As well as this marvelous array of materials, I also have access to a small bluebird mixer and a larger dough mixer for preparing clay. The larger mixer can do 200-300lbs in one go and the smaller one is more suited to 50-100lbs. Okay, so that’s the clay part… I want to test and see how they respond to soda firing.
WHY SODA?
As an apprentice and afterwards in North Carolina, I primarily fired my pots in wood kilns with the late addition of salt. The salt (NaCl) vaporises and sodium molecules bond with silica in the clay to produce a clear glaze on the pots. I love the surfaces that result from this atmosphere, but feel like I have a pretty good handle on it as a process.
Soda firing, however, is a whole other thing. The process is similar to firing with salt, but instead of sodium chloride, you introduce soda ash (Na₂CO₃) or baking soda (NaHCO₃). The sodium still reacts with the clay to form a clear glaze on the pots, but it is much less volatile. With salt firing you rarely get a dry surface; it flies so readily around the kiln reacting with the pots. With soda you get much more localized effects; each pot will have more soda on one side than the other. This makes for more dramatic surfaces.
Here’s what I mean. Below on the left is a salt glazed jar… the orange peel effect of the salt wraps around the whole pot in a pretty even way. The variation on the belly/shoulder of the pot is due to a light dusting of wood ash, not the salt. On the right is a soda glazed cannister jar out of my first soda firing. The area that got a lot of soda went grey and the area with less went red. There was a clay slip on each of these pots. You can see the darker clay color underneath the salt glazed jar and the lighter clay under the slip on the soda jar. This shows how much the clay is crucial in soda firing. The slip here is registering the level of soda far more than the white stoneware underneath would.
Salt glazed cannister jar, Hamish Jackson, fired in Lara O’Keefe’s salt kiln, 2019, NC.
Soda glazed cannister jar, Hamish Jackson, fired in the soda kiln at USU, 2020, UT.
I have wanted to delve into soda for a while now, but never had a real chance. This goal aligned with my other one of testing clays. I figured why not experiment with the available clays in the studio in the soda kiln. By doing so I would see how reactive they are and to try to understand their character.
So for my first soda firing, I made a bunch of test tiles and some pots using a couple of clays recommended by Dan Murphy (one of the professors). I chose to run each slip test on 3 different clay bodies to see what difference that made. I used a high alumina porcelain, a white stoneware and an iron rich clay. Each test got 3 trials in the kiln. In retrospect, I wish I had made twice the amount of tiles so I could have saved a whole set for my next firing. But I got plenty of information anyway. It was an excellent exercise.
Before loading and firing, I spoke with several people about how they have approached soda: Casey Beck, Harry Levenstein, Louis Reilly, Denise O’Connell Joyal and Isaac Howard. It was super helpful to pick their brains before I got rolling. Check them out, they all make really lovely pots.
THE FIRING
I decided to fire in the way I thought would produce the best results (after much thought). I did an hour of body reduction when cone 010 went down, keeping the kiln in medium reduction on the way up and then sprayed in soda slowly over the course of 6 hours, starting when cone 7 went down, ending when cone 10 was down. I went in cycles, pushing the damper in when I introduced soda, with a couple of pieces of wood each time too, and then opened the damper back up to get the temp to rise back to where it had been. At the end of the firing, I let the kiln drop to 1850°F and then down-fired it in reduction to 1600°F. This was fairly time consuming and I was happy to have Tansy O’Bryant on the crew. She had pots in the kiln and was very helpful with the down-firing and clean up afterwards.
Alright, enough chatter. Here’s some of the results…
NOTE ON THE RESULTS
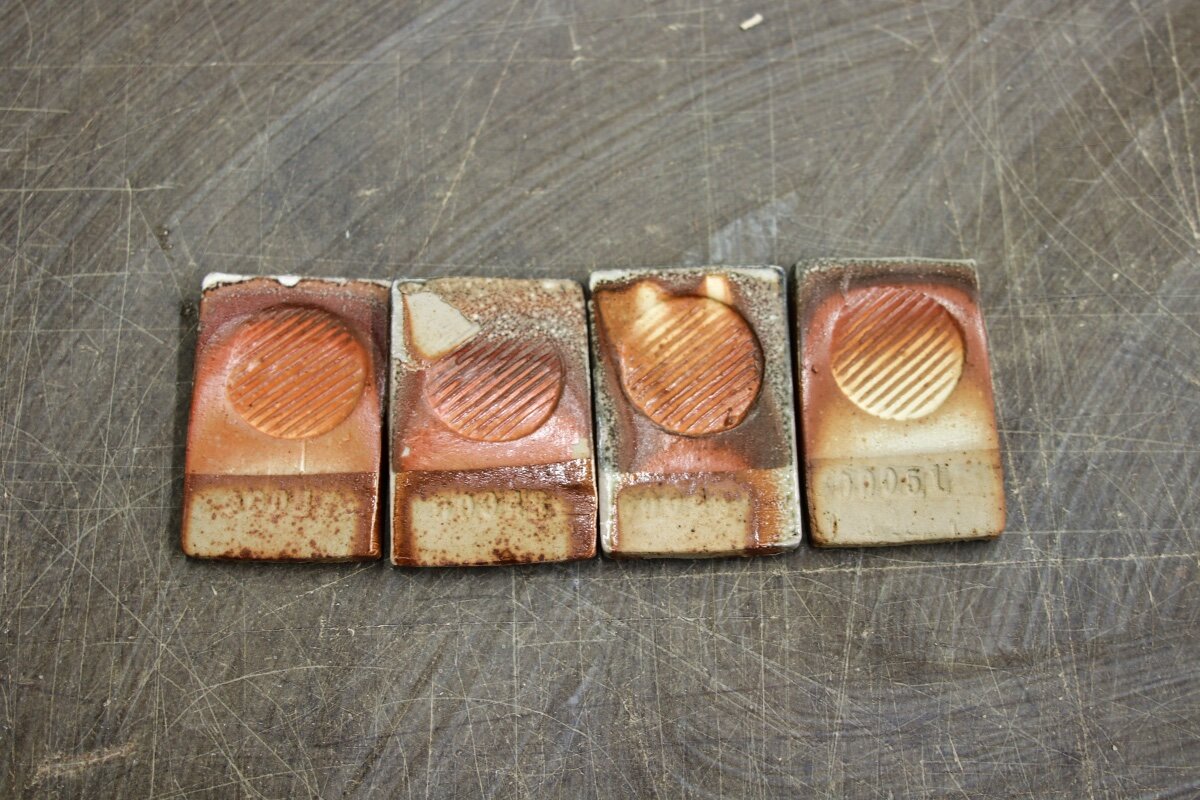
Some of the tests are based on a basic slip recipe (#6) with additions. #6 is 50% Helmar Kaolin : 50% OM4. Each test was dipped once to cover about 3/4 of the tile and then dipped again on the top 1/3. The tiles were packed in the kiln pretty tightly so often they only got dosed with soda on the edge/top corner. Most of the information about how the slip will act in soda is shown in these tight bands/gradients near the top of the tiles. I spent a lot of time staring at these tight lines of color to discern the results. Some of it is translatable in these pictures, but there is no substitute for handling the actual tiles. If only we had holographic technology!
Each set of test tiles fit on a single shelf (12” x 24”). To easily keep track, I put the white stoneware tests up top, the iron rich stoneware in the middle and the porcelain down low.
I recommend clicking one of the images to open a pop up window to view them.
The kiln was not entirely full of test tiles. Below are some pics of the kiln as I unloaded it…




























Here are a few pictures of pots that were dipped in a couple of different slips. You get a much better feel for what the slips might do seeing them on a form rather than a test tile.
Finally, here’s the firing schedule and my initial notes from how it went (just in case I lose my notebook or someone burns it). I have summarized the notes below, so no need to zoom in and try and read my dodgy handwriting haha.
RESULTS/NOTES/CONCLUSIONS:
I will end with just some quick notes of the results:
Clay is of utmost importance. This was clearly shown by the test tiles. The iron rich clay was the most impacted by the reduction cooling (deep purples and reds). It was the least reactive/flashy, though. The porcelain flashed the brightest.
The white stoneware is definitely best for having slips applied over it. Many of them flaked off the porcelain and the stoneware is a white enough background to allow bright colorful slip action.
The porcelain on its own without a slip could be beautiful. Some of my favorite results were simply the plain porcelain clay ice cream and cereal bowls…
Brighter color where less soda: Heavy soda tends towards light or dark grey.
Neph sye brightens colors and flashes well. As you increase the quantity of it in a slip it fluxes it more and the flashing lines get more blurry'/less defined (above 35%).
Talc might be an interesting addition to a clay body: For color response and resist the soda.
PACK THE KILN BETTER! Try to use less shelves and stagger the pots on each shelf to allow soda to travel around more easily.
Placing pots very close together produced flashing, but you have to let the flame and soda get there.
Not sure how much of an effect the reduction cool had. Also not sure how much the wet wood that I introduced (water) affected the results.
Used 10lbs soda, all sprayed in to the fireboxes. The middle of the kiln was pretty dry, the top and bottom got a good bit of soda.
* * *
Thanks for reading. Especially if you got all the way this far reading it all! Happy Holidays to you and yours! I’ll be posting about my #2 SODA FIRING in the next day or so.
Opening Reception of the Juried Functional Teapot Show, Black Iris Gallery, Richmond 2020
I wanted to share a few of the photos from the opening reception, before the gallery closed (rightly so) due to the coronavirus pandemic.
The writing on the wall…
One lady came in with extremely cold hands. Tea was the perfect solution! She also may have put two teacups up her top…
I had surveys/questionnaires out for people to fill out which teapots they liked the look of the most and which they thought would be the best functionality-wise. The completed surveys went in a beautiful pot made by Chad Brown (a 3rd generation Seagrove potter). Chad also kindly let me take the wood used for the shelves and tables from his grandad’s old barn that had recently fallen down.
We drank some glorious tea courtesy of our sponsor Teaism, a wonderful tea shop in Washington, DC. That night we drank mostly a lavender and mint blend that they make. Everyone thought it was delicious. I even turned some ardent non-tea-drinkers on to it.
Here are some more pics of the wood-fired teacups I made and the writing on the wall about them…


Here’s one final picture. This guy was great!
The Teapots of the Inaugural Juried Functional Teapot Show
Before the Corona Virus really took hold I was able to set up the teapot show and have it on display for a couple of weeks. Here’s the pots as they sat in the gallery.
Thanks for taking a look! I plan on bringing the Juried Functional Teapot Show back next year with all new teapots… it will be up in Cincinnati Ohio next March NCECA 2021, so hope to see some of you there!